The Devil’s Burden (Devilish Diseases Part 1)
The Tasmanian Devil's Struggle Against Transmissible Tumors
Introduction: Transmissible Cancers — Nature’s Exceptionally Rare Horror Story
Cancer is typically seen as a disease of the individual, but transmissible cancers like Tasmanian Devil Facial Tumor Disease (DFTD) turn this notion on its head. Unlike most cancers, these can be spread directly between individuals like an infection. The discovery of DFTD in the 1990s was a shocking revelation, unveiling a rare form of cancer capable of passing from one devil to another through biting. This mechanism is unlike virus-driven cancers in humans (e.g., HPV-associated cancers) because it involves the direct transmission of living tumor cells rather than a viral agent.

Origins and Evolution: The Unfolding Story of DFT1 and DFT2
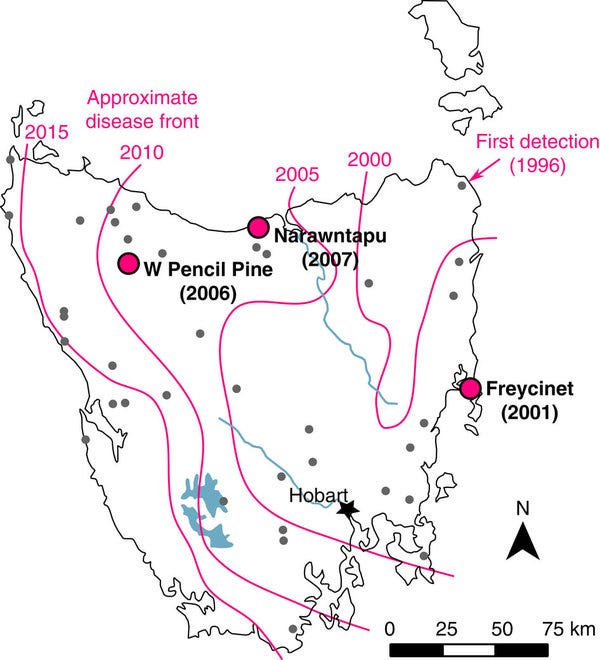
Initially detected in 1996, DFTD quickly became a critical concern for conservationists. Early research, such as Pearse & Swift’s 2006 work, identified DFTD as an allograft, meaning the tumor cells themselves are transplanted from one devil to another. The disease has swiftly spread across 95% of the Devil’s range in just two decades (Raven et al., 2024). This unique characteristic set the stage for further investigation into its cellular origins. By 2010, Murchison et al. pinpointed the Schwann cell origin of the cancer, using advanced transcriptome sequencing to identify myelin-related proteins like periaxin (PRX) and myelin protein zero (MPZ). This discovery highlighted the tumor’s specialized cell lineage, providing a new avenue for understanding how DFTD evades the host immune system.
However, the plot thickened in 2014 when a second lineage, DFT2, was identified. Unlike DFT1, which had spread rapidly across Tasmania, DFT2 appeared to be localized, emerging in distinct populations. The independent evolution of DFT2 suggests strong selective pressures in the devil population, driven by low genetic diversity and frequent aggressive interactions. This dual threat complicates conservation efforts, as it indicates the potential for ongoing emergence of new transmissible tumor lineages.
Expanding the Molecular Mechanisms and Tumor Biology
The immune evasion strategies of DFTD are a fascinating area of study. Kreiss et al. (2016) demonstrated how DFTD downregulates MHC class I molecules, effectively hiding from the devil’s immune defenses. The tumor’s expression of Schwann cell markers like nestin (NES) and nerve growth factor receptor (NGFR) further complicates immune detection. These proteins, typically found in peripheral nerve tissues, act as a cellular disguise, allowing the cancer cells to blend in and avoid immune attack.
Interestingly, DFT2 has shown a different pattern of immune interaction. Research indicates that DFT2 may have a slightly higher MHC expression, suggesting an evolutionary adaptation to increased immune recognition in certain devil populations (James et al., 2019; Siddle et al., 2013). This difference between DFT1 and DFT2 provides a unique opportunity to study the co-evolutionary dynamics of cancer and host, offering potential insights for broader cancer research.
Comparative Analysis: DFTD, CTVT, and Other Transmissible Cancers
Transmissible cancers are a rare phenomenon, with only a handful of documented cases across species. Tasmanian Devil Facial Tumor Disease (DFTD) stands out due to its aggressive nature and impact on a vulnerable species. However, examining other transmissible cancers like Canine Transmissible Venereal Tumor (CTVT) and contagious sarcomas in hamsters provides critical context for understanding DFTD’s unique characteristics.
Canine Transmissible Venereal Tumor (CTVT): The Oldest Known Transmissible Cancer
CTVT is thought to be over 10,000 years old, making it the oldest known transmissible cancer (Jones et al., 2015). Unlike DFTD, which spreads through biting, CTVT is transmitted via sexual contact. This cancer has evolved a relatively stable relationship with its hosts, often regressing spontaneously after a few months (Rebbeck et al., 2009). CTVT’s evolutionary success lies in its ability to coexist with its host without causing severe harm, balancing transmissibility and host survival. This adaptation is a stark contrast to DFTD, which often kills its host within a year of infection.
CTVT’s stability over millennia highlights a key evolutionary strategy: avoiding rapid host death to ensure continued transmission. The tumor modulates local immune responses, suppressing immune attacks in the reproductive tract where it thrives. Murchison et al. (2014) found that CTVT has a remarkably low mutation rate, suggesting that its ancient lineage has achieved a stable genetic configuration suited for long-term survival in the canine population.
HPV-Related Cancers: A Viral Perspective on Transmissibility
While not a transmissible cancer in the strictest sense, HPV provides an interesting comparison. Human papillomavirus is a sexually transmitted virus that can lead to cancers like cervical cancer. Unlike DFTD or CTVT, where the cancer cells themselves are transmitted, HPV spreads as a virus that later induces oncogenesis in the host cells (Sabatini and Choicca., 2019). The evolutionary strategy of HPV differs significantly, relying on viral replication rather than direct cell transmission, yet it blurs the line between direct and virus-mediated transmissible cancers. The oncogenic potential of HPV provides insights into how infectious agents can drive cancer evolution and offers a comparative framework for studying the dynamics of DFTD.
Conservation Strategies and Ongoing Research: Balancing Innovation and Practicality
Conserving the Tasmanian devil population in the face of DFTD requires a multifaceted approach. Early strategies focused on culling infected individuals, but this method proved unsustainable due to the rapid and aggressive spread of the disease before visible symptoms appeared (Beeton and McCallum, 2011; Lachish et al., 2010). As a result, the focus shifted towards more innovative and sustainable solutions.
Vaccination Trials and Immunotherapy Efforts
One of the most promising approaches has been the development of vaccines. Pye et al. (2018) led trials using autologous tumor cells to stimulate an immune response. Early results showed some success, with vaccinated devils displaying a delayed onset of the disease. However, logistical challenges like capturing and vaccinating wild devils have limited the scalability of this approach. Future research may involve developing oral vaccines, which could be distributed in bait form, similar to rabies vaccination programs in wildlife.
Immunotherapy, which has revolutionized human cancer treatment, is another area of active exploration. Researchers are investigating immune checkpoint inhibitors that could potentially enhance the devil’s immune response against DFTD. This approach leverages insights from human oncology, adapting treatments that have shown promise in combating aggressive cancers like melanoma.
The Role of Zoos and Captive Breeding Programs
Captive breeding programs have played a crucial role in preserving genetic diversity and preventing the extinction of Tasmanian devils. Zoos across Australia have been integral in maintaining insurance populations, with a focus on breeding individuals that are free of DFTD. These programs have successfully reintroduced healthy devils into the wild, though the long-term success of these efforts depends on continued monitoring and genetic management.
Researchers have also explored the possibility of “assisted colonization,” where devils are introduced to new, uninhabited areas of Tasmania to establish disease-free populations (Global Wildlife Conservation). This strategy carries its own risks, including the potential for unforeseen ecological impacts, but it represents a proactive approach to conservation in the face of an uncertain future. A successful trial of assisted colonization took place with 15 Devils being moved to mainland Australia in 2020 with the population seeming to thrive there after their 3000 year absence from the island.
Long-Term Ecological Impacts: The Ripple Effect of DFTD
The decline of Tasmanian devils due to DFTD has profound and far-reaching consequences for the ecosystem. As apex scavengers, devils help control populations of smaller carnivores like feral cats and foxes. With fewer devils, these mesopredators have experienced population booms, leading to increased predation on native birds, marsupials, and other small mammals. This phenomenon, known as mesopredator release, has destabilized prey populations, driving some species closer to extinction.
Recent studies have shown that the decline of devils also impacts nutrient cycling. Tasmanian devils play a crucial role in breaking down carrion, returning essential nutrients to the soil. With fewer scavengers, carcasses decompose more slowly, altering soil composition and potentially affecting plant growth and forest dynamics. This shift in nutrient cycling can have cascading effects, disrupting the entire food web.
Additionally, the decline in devil populations has created a feedback loop that exacerbates their own vulnerability. As feral cats and foxes become more dominant, they outcompete devils for food resources and introduce diseases like toxoplasmosis, which further weaken devil populations. Conservationists are now grappling with the challenge of restoring this delicate balance, exploring interventions like habitat restoration and predator control programs.
Future Directions in Transmissible Cancer Research: Beyond the Devils
The study of DFTD has opened new avenues for research, not only in wildlife conservation but also in human oncology. One of the most promising areas of investigation is the genetic basis of resistance. Raven et al. (2024) have identified specific genetic variants that may confer added risk to certain individuals. By understanding these genetic factors, researchers in the future may look to develop targeted breeding programs or even gene-editing interventions using CRISPR. This could pave the way for innovative conservation strategies that enhance the resilience of devil populations.
There is also a growing interest in the potential for immunotherapy in wildlife cancer treatment. Building on successes in human cancer treatment, researchers are exploring immune checkpoint inhibitors and personalized vaccines tailored to the unique genetics of individual devils. These therapies could help boost the immune response against DFTD, offering a new tool in the fight against the disease.
The Broader Implications for Cancer Evolution
The case of DFTD provides a unique window into the evolutionary dynamics of cancer. Unlike typical cancers that arise within an individual, transmissible cancers like DFTD must adapt to survive across multiple hosts. This requires a delicate balance between aggressive growth and immune evasion. The study of DFTD and other transmissible cancers could yield insights into how cancers evolve in response to selective pressures, potentially informing new approaches to human cancer treatment.
Conclusion: The Legacy of Tasmanian Devil Facial Tumor Disease
Tasmanian Devil Facial Tumor Disease is more than a conservation crisis—it’s a biological anomaly that challenges our understanding of cancer. The emergence of DFTD has forced scientists to rethink the boundaries between infectious disease and oncology, offering unprecedented insights into cancer evolution. As we continue to study this phenomenon, we gain not only a better understanding of how to protect the Tasmanian devil but also valuable knowledge that could transform human cancer research.
The fight against DFTD is far from over, but the lessons learned from this battle could shape the future of cancer treatment, wildlife conservation, and our understanding of evolutionary biology. It’s a stark reminder of the interconnectedness of all life and the unexpected ways in which nature continues to surprise us.
Citations and Further Reading on DFTD and Transmissible Cancers
1.
Bramwell G, DeGregori J, Thomas F, Ujvari B. Transmissible cancers, the genomes that do not melt down. Evolution. 2024;78(7):1205-1211. doi:10.1093/evolut/qpae063
2.
Bruno C, Comar T, Powell M, Tameklo A. Age-Structured and Vaccination Models of Devil Facial Tumor Disease. Spora: A Journal of Biomathematics. 2017;3(1). doi:http://doi.org/10.30707/SPORA3.1Bruno
3.
Cheng Y, Makara M, Peel E, Fox S, Papenfuss AT, Belov K. Tasmanian devils with contagious cancer exhibit a constricted T-cell repertoire diversity. Commun Biol. 2019;2(1):1-9. doi:10.1038/s42003-019-0342-5
4.
Cunningham CX, Comte S, McCallum H, et al. Quantifying 25 years of disease-caused declines in Tasmanian devil populations: host density drives spatial pathogen spread. Ecology letters. 2021;24(5):958. doi:10.1111/ele.13703
5.
Deakin JE, Bender HS, Pearse AM, et al. Genomic Restructuring in the Tasmanian Devil Facial Tumour: Chromosome Painting and Gene Mapping Provide Clues to Evolution of a Transmissible Tumour. PLOS Genetics. 2012;8(2):e1002483. doi:10.1371/journal.pgen.1002483
6.
Epstein B, Jones M, Hamede R, et al. Rapid evolutionary response to a transmissible cancer in Tasmanian devils. Nat Commun. 2016;7(1):12684. doi:10.1038/ncomms12684
7.
Flies AS, Lyons AB, Corcoran LM, et al. PD-L1 Is Not Constitutively Expressed on Tasmanian Devil Facial Tumor Cells but Is Strongly Upregulated in Response to IFN-γ and Can Be Expressed in the Tumor Microenvironment. Front Immunol. 2016;7. doi:10.3389/fimmu.2016.00581
8.
James S, Jennings G, Kwon YM, et al. Tracing the rise of malignant cell lines: Distribution, epidemiology and evolutionary interactions of two transmissible cancers in Tasmanian devils. Evolutionary Applications. 2019;12(9):1772-1780. doi:10.1111/eva.12831
9.
Jones EA, Cheng Y, Belov K. The origin, dynamics, and molecular evolution of transmissible cancers. AGG. 2015;5:317-326. doi:10.2147/AGG.S61298
10.
Kosack L, Wingelhofer B, Popa A, et al. The ERBB-STAT3 Axis Drives Tasmanian Devil Facial Tumor Disease. Cancer Cell. 2019;35(1):125. doi:10.1016/j.ccell.2018.11.018
11.
Kreiss A, Brown GK, Tovar C, Lyons AB, Woods GM. Evidence for induction of humoral and cytotoxic immune responses against devil facial tumor disease cells in Tasmanian devils (Sarcophilus harrisii) immunized with killed cell preparations. Vaccine. 2015;33(26):3016-3025. doi:10.1016/j.vaccine.2015.01.039
12.
Kwon YM, Gori K, Park N, et al. Evolution and lineage dynamics of a transmissible cancer in Tasmanian devils. PLoS Biology. 2020;18(11):e3000926. doi:10.1371/journal.pbio.3000926
13.
Lachish S, McCALLUM H, Mann D, Pukk CE, Jones ME. Evaluation of Selective Culling of Infected Individuals to Control Tasmanian Devil Facial Tumor Disease. Conservation Biology. 2010;24(3):841-851. doi:10.1111/j.1523-1739.2009.01429.x
14.
Metzger MJ, Reinisch C, Sherry J, Goff SP. Horizontal transmission of clonal cancer cells causes leukemia in soft-shell clams. Cell. 2015;161(2):255. doi:10.1016/j.cell.2015.02.042
15.
Murchison EP, Tovar C, Hsu A, et al. The Tasmanian Devil Transcriptome Reveals Schwann Cell Origins of a Clonally Transmissible Cancer. Science (New York, NY). 2010;327(5961):84. doi:10.1126/science.1180616
16.
Murchison EP, Wedge DC, Alexandrov LB, et al. TRANSMISSIBLE DOG CANCER GENOME REVEALS THE ORIGIN AND HISTORY OF AN ANCIENT CELL LINEAGE. Science (New York, NY). 2014;343(6169):437. doi:10.1126/science.1247167
17.
Ní Leathlobhair M, Lenski RE. Population genetics of clonally transmissible cancers. Nat Ecol Evol. 2022;6(8):1077-1089. doi:10.1038/s41559-022-01790-3
18.
Pearse AM, Swift K. Transmission of devil facial-tumour disease. Nature. 2006;439(7076):549-549. doi:10.1038/439549a
19.
Pye R, Hamede R, Siddle HV, et al. Demonstration of immune responses against devil facial tumour disease in wild Tasmanian devils. Biology Letters. 2016;12(10):20160553. doi:10.1098/rsbl.2016.0553
20.
Pye R, Patchett A, McLennan E, et al. Immunization Strategies Producing a Humoral IgG Immune Response against Devil Facial Tumor Disease in the Majority of Tasmanian Devils Destined for Wild Release. Frontiers in Immunology. 2018;9:259. doi:10.3389/fimmu.2018.00259
21.
Raven N, Klaassen M, Madsen T, et al. Complex associations between cancer progression and immune gene expression reveals early influence of transmissible cancer on Tasmanian devils. Front Immunol. 2024;15:1286352. doi:10.3389/fimmu.2024.1286352
22.
Rebbeck CA, Thomas R, Breen M, Leroi AM, Burt A. Origins and evolution of a transmissible cancer. Evolution. 2009;63(9):2340-2349. doi:10.1111/j.1558-5646.2009.00724.x
23.
Sabatini ME, Chiocca S. Human papillomavirus as a driver of head and neck cancers. British Journal of Cancer. 2019;122(3):306. doi:10.1038/s41416-019-0602-7
24.
Tasmania U of. Importance of saving the Tasmanian devil. University of Tasmania. June 14, 2022. Accessed November 26, 2024. https://www.utas.edu.au/community-and-partners/giving/the-tasmanian-devil-appeal/tabs/importance-of-saving-the-tasmanian-devil
25.
Ujvari B, Papenfuss AT, Belov K. Transmissible cancers in an evolutionary context. BioEssays. 2016;38(S1):S14-S23. doi:10.1002/bies.201670904



