Finding Risk Where History Put It: Founder Effects, Population Bottlenecks, Rare Diseases, and Screening Programs
Opening Disclaimer: I’m not a geneticist. Any errors here are my own and I’d appreciate corrections from geneticists in the comments. They’re more than welcome! I’m approaching this as an epidemiologist trying to explain how population history can make “rare” diseases locally common, along with how that shapes screening and prevention practices.
When “rare” isn’t rare
Most people hear the term “rare disease” and think of some sort of needle in a haystack problem. And while that’s often true at the national level, and especially for researchers looking to find multiple people with a rare disease to treat, but at the scale where people actually form their families (regions, villages, endogamous communities) history can flip the script. A population bottleneck or serial founder events can make a rare, recessive condition (globally) locally common due to things like drift, isolation, and endogamy concentrating specific variants over the generations.
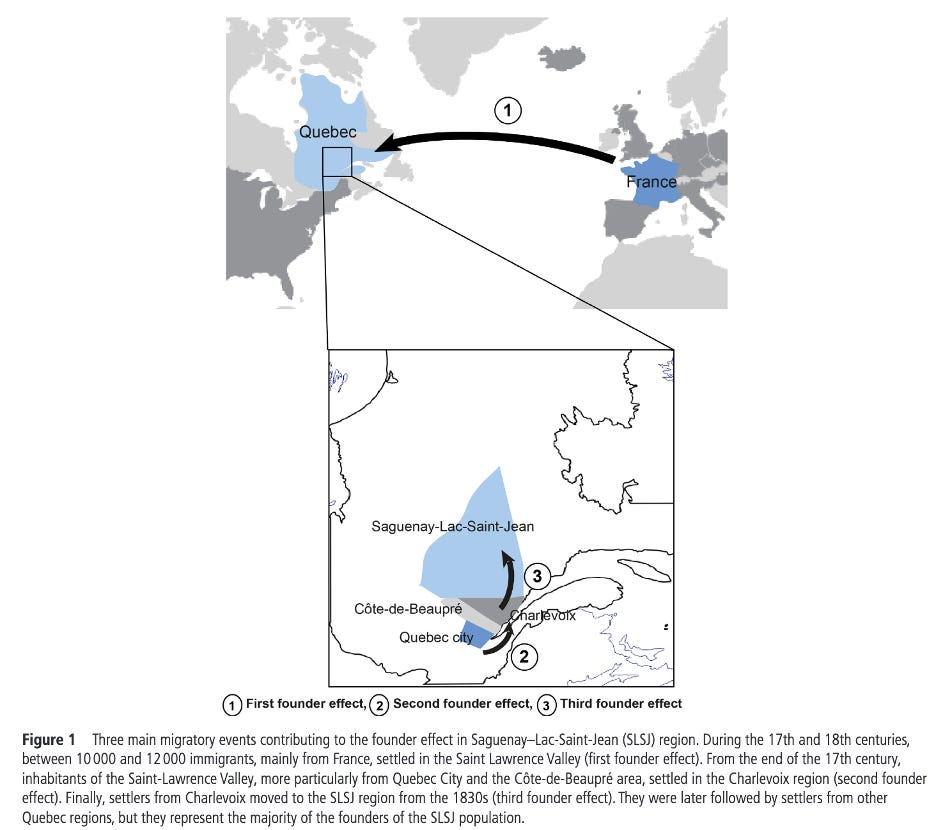
Quebec’s Saguenay-Lac-Saint-Jean (SLSJ) region is a great example of this. A handful of 17th-18th century founders, combined with a subsequent geographic isolation and large family sizes ended up producing a region where a small set of severe, recessive diseases (tyrosinemia type I, ARSACS, Leigh syndrome, and Andermann syndrome, among others) occur far more often than in more outbred populations1. The map above from Bchetnia et al (2021) shows the three identified founder effects in the region and when they happened. Instead of shrugging off the risk, SLSJ built a community-informed carrier-screening program where residents are screened, carriers are identified, and at-risk couples are counseled2 resulting in a founder-aware public health program that prevents suffering down the road.
But let’s zoom out a bit. What happened in SLSJ is far from an anomaly. Island populations like those of Tristan da Cunha, major diasporas like Ashkenazi Jews, and entire regions like Finland deal with founder structure impacts, with the latter turning into its own discovery engine through the FinnGen program3,4. There’s also the under appreciated story in South Asia, where dozens of large endogamous groups show founder events at least as strong as the Finnish or Ashkenazi examples and, as a result, much higher local burdens of specific recessive diseases5,6. Even incredibly diverse cities like New York see founder-enriched pathogenic variance in under-represented communities via health-system genomics7.
Mechanism 101: bottlenecks, drift, endogamy, and consanguinity
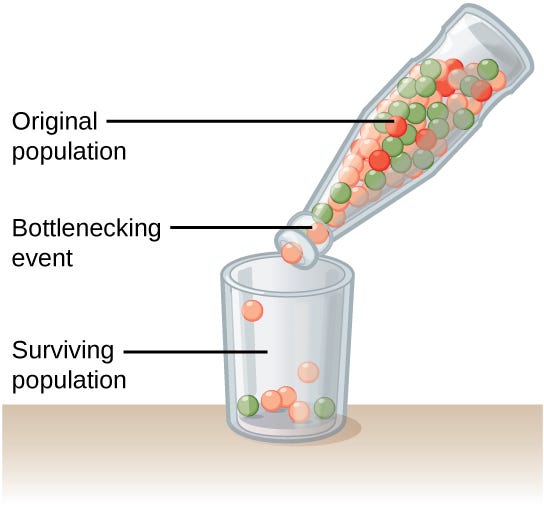
Founder effect and bottleneck. A founder effect happens when a new population starts from a small number of ancestors; a bottleneck is the same idea in mid-stream where population size collapses temporarily, then rebounds After a bottleneck or a small founder event, random drift can push specific alleles to much higher frequency than in the source population. If a deleterious recessive variant survives the bottleneck, the homozygote rate scales with the square of its frequency, so small frequency bumps can create large disease burdens locally in the population1.
Endogamy vs consanguinity. Endogamy is marrying within a culturally or geographically defined group; consanguinity is marrying a close relative. Both increase the chance that offspring inherit the same ancestral haplotype from both parents, but they do it via different routes. Endogamy acts over many generations by restricting the mating pool; consanguinity acts in a single generation by increasing close-kin unions. In practice they can co-occur. The key point for epidemiology: both increase the fraction of the genome in long runs of homozygosity (ROH), which raises the probability of homozygous recessive disease, especially for variants that drifted to higher frequency during founder events8.
How we measure it now. Modern tools make founder effects quantifiable rather than hand-wavy.
• Runs of homozygosity (ROH) & Identity by descent (IBD): Using genome-wide SNP data, we can identify long ROH (identical alleles from both parents across megabase spans) and identity-by-descent (IBD) segments shared between individuals. Long ROH are footprints of small effective population size and endogamy; they localize recessive loci and correlate with recessive disease burden8.
• Timing & strength of founder events: Newer methods like ASCEND reconstruct when and how strong founder events were by modeling allele sharing across the cohort, converting population history into testable numbers9. That matters because a recent, strong founder event yields longer ROH and higher near-term disease risk than an ancient, weak one.
• Biobank-scale nuance: At scale, we also learn that “recessive” rarely means “no effect unless one has both alleles.” Many pathogenic or likely pathogenic alleles show measurable heterozygote effects on traits and disease risk, which means additive-only models undercount the burden in founder contexts10).
Why prevalence can jump so much. Put these pieces together: a deleterious recessive variant at frequency p produces a homozygote frequency of ~p² under random mating. If p drifts from 0.1% to 1% in a founder population, p² jumps from ~1 in a million to ~1 in ten thousand. Layer endogamy (and sometimes consanguinity) on top, and you push the realized homozygote rate even higher than p² would predict in a panmictic population. That’s why SLSJ can have orders-of-magnitude higher prevalence for specific conditions than neighboring regions, and why South Asian founder groups show dramatically elevated counts of rare homozygotes compared with outbred cohorts1,6.
What this doesn’t mean. None of this implies “genetic purity,” racial essentialism, or moral judgment. Founder effects are demographic artifacts that show up wherever small, semi-isolated groups exist, such as religious communities, islands, mountain valleys, colonial settlements, even neighborhoods within megacities. The public health play is to recognize the pattern and respond with voluntary, community-informed screening and counseling, not stigma7.
South Asia & diaspora
I mentioned earlier that a more under appreciated founder-effect story is that of South Asia. A couple of lines of evidence stand out here. First is the population-genetic structure, which has multiple endogamous groups showing strong founder effects, with some stronger than the more canonical Finnish/Ashkenazi examples (due to long-standing social endogamy with the occasional consanguinity thrown in as well)5. One example was the identification of butyrylcholinesterase defiency in the Vysya community (which contraindicates certain anaesthetics). Second are the clinical cohorts made up of hospital-based datasets from India and Pakistan that show high rates of homozygosity and elevated burdens of rare, likely deleterious genes. The exact type of architecture that makes recessive diseases preventable with the right type of carrier-screening and genetic counseling programs6.
One paper by Wall et al6 goes a bit further than pointing out the structure by quantifying how homozygote counts scale with measured inbreeding/endogamy and show the dispersion across groups. The practical read is that founder-aware designs will surface recessive disease genes efficiently, pan-ethnic expanded carrier screening is a baseline, but augmented founder panels for specific groups can be additive where carrier frequencies are high, and diaspora care (e.g., in the US/UK) needs to stop assuming that “South Asian” is a homogeneous grouping5,6. The upside here is real, all one needs to do is look at the Ashkenazi, SLSJ programs, and other targeted programs to show that community-led screening can reduce burden2,5.
Hidden founders in diverse cities
Using IBD analysis on data from over 25,000 residents of New York City from the All of Us study and Mount Sinai’s BioMe biobank, Isshiki and colleagues identified 16 groups with recent shared ancestry living in NYC, with 8 defined as founder populations from under-represented communities (in terms of medical genomics research) including Puerto Rican, Garifuna, Filipino, Ashkenazi, and Romani7. Within those groups, they found multiple founder enriched pathogenic variants, 25 of which met the carrier frequency (1 in 100) to justify for inclusion on what’s called a Tier-2 carrier panel and had never been reported before. But that’s not the headline. Anyone who knows about NYC knows it’s had plenty of founding groups over time. It’s that the standard ethnicity-labeled panels missed several variants because the ancestral labels weren’t capturing real demography11.
This type of IBD grouping can be operationalized by health systems (as soon as more of them, and their patients, get on board with genetic screening). Any system with genomic data can detect these IBD clusters and work with community partners to build in opt-in screening and counseling.
How Finland turned its founder history into discovery
Finland is a clean example of how demographic history can become a research accelerator rather than a curiosity. Because of past bottlenecks and relative isolation, many deleterious alleles that are vanishingly rare elsewhere sit at higher frequency in Finns, including those for congenital nephrotic syndrome, Meckel syndrome, progressive myoclonic epilepsy, and about 40 other diseases (all canonically catalogued in Uusimaa et al (2022). FinnGen ties that founder structure to national health registers at scale, which means low-frequency coding variants show up with enough cases to map disease and trait associations that are hard to see in outbred cohorts3. The “Finnish Disease Heritage” review shows the other half of the loop: decades of clinical genetics in an isolated population shorten diagnostic odysseys and clarify natural history across a set of recurrent recessive disorders4. Even outside classic “rare disease,” this architecture helps surface drug targets and treatment signals because ascertainment and longitudinal follow-up are built into the registries3,12. Finland shows just how any place with strong founder events can convert that structure into discovery and better care if it links genomes to reliable clinical data and does the work.
Where I land on implementation
I am pro-implementation of founder/consanguinity-aware screening programs when the burden is real. And while I’d like it to be the default, that may not be feasible in some populations. That said, the Saguenay-Lac-Saint-Jean experience shows you can normalize carrier screening, counsel identified carriers and at-risk couples, and prevent suffering without turning this into a labeling exercise. The New York City work adds a reminder that founder burdens exist in diverse urban populations and that grouping by identity-by-descent can reveal risks standard ethnicity labels miss, which argues for thoughtful, opt-in offers and clear counseling rather than gatekeeping. But again, I am not a geneticist, and this is not a policy manifesto. It is an epidemiologist’s read of the evidence. And what I see is that founder effects make some “rare” diseases common in specific places and groups, and well-designed, community-informed screening programs are a practical way to reduce that burden for the future.
Updates/Errata. I’ll note corrections and substantial clarifications here with dates and attributions.
• 2025-08-27 [none yet]
Citation
1. Bchetnia M, Bouchard L, Mathieu J, et al. Genetic burden linked to founder effects in Saguenay–Lac-Saint-Jean illustrates the importance of genetic screening test availability. J Med Genet. 2021;58(10):653-665. doi:10.1136/jmedgenet-2021-107809
2. Fortin CA, Côté-Richer M, Truchon K, et al. Successes of an innovative population-based carrier screening program for 4 prevalent recessive hereditary diseases in a population with a founder effect in Quebec, Canada. Genet Med Open. 2025;3. doi:10.1016/j.gimo.2025.103435
3. Kurki MI, Karjalainen J, Palta P, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613(7944):508-518. doi:10.1038/s41586-022-05473-8
4. Uusimaa J, Kettunen J, Varilo T, et al. The Finnish genetic heritage in 2022 – from diagnosis to translational research. Dis Model Mech. 2022;15(10):dmm049490. doi:10.1242/dmm.049490
5. Nakatsuka N, Moorjani P, Rai N, et al. The promise of disease gene discovery in South Asia. Nat Genet. 2017;49(9):1403-1407. doi:10.1038/ng.3917
6. Wall JD, Sathirapongsasuti JF, Gupta R, et al. South Asian medical cohorts reveal strong founder effects and high rates of homozygosity. Nat Commun. 2023;14(1):3377. doi:10.1038/s41467-023-38766-1
7. Isshiki M, Griffen A, Meissner P, et al. Genetic disease risks of under-represented founder populations in New York City. medRxiv. Published online September 28, 2024:2024.09.27.24314513. doi:10.1101/2024.09.27.24314513
8. Naseri A, Zhi D, Zhang S. Discovery of runs-of-homozygosity diplotype clusters and their associations with diseases in UK Biobank. Gao Z, Weigel D, Carmi S, eds. eLife. 2024;13:e81698. doi:10.7554/eLife.81698
9. Tournebize R, Chu G, Moorjani P. Reconstructing the history of founder events using genome-wide patterns of allele sharing across individuals. PLoS Genet. 2022;18(6):e1010243. doi:10.1371/journal.pgen.1010243
10. Heyne HO, Karjalainen J, Karczewski KJ, et al. Mono- and biallelic variant effects on disease at biobank scale. Nature. 2023;613(7944):519-525. doi:10.1038/s41586-022-05420-7
11. Isshiki M, Griffen AJ, Meissner P, et al. Genetic disease risks of under-represented founder populations in New York City. PLoS Genet. 2025;21(6):e1011755. doi:10.1371/journal.pgen.1011755
12. Kiiskinen T, Helkkula P, Krebs K, et al. Genetic predictors of lifelong medication-use patterns in cardiometabolic diseases. Nat Med. 2023;29(1):209-218. doi:10.1038/s41591-022-02122-5